
Chemistry, 15.06.2021 18:20 aliciabenitez
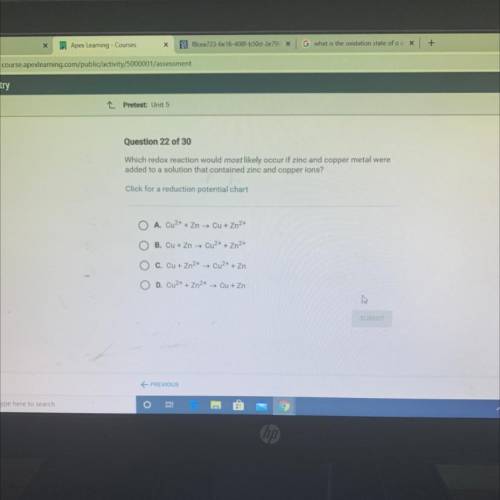
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that contained zinc and copper ions?
Click for a reduction potential chart
A. Cu2+ + Zn → Cu + Zn2+
O B. Cu + Zn → Cu2+ + Zn2+
C. Cu + Zn2+ → Cu2+ + Zn
O D. Cu2+ + Zn2+ → Cu + Zn

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Which redox reaction would most likely occur if zinc and copper metal were
added to a solution that...
Questions

Computers and Technology, 16.07.2019 21:30

Computers and Technology, 16.07.2019 21:30

Advanced Placement (AP), 16.07.2019 21:30






History, 16.07.2019 21:30



Spanish, 16.07.2019 21:30

English, 16.07.2019 21:30

Physics, 16.07.2019 21:30

Health, 16.07.2019 21:30








