Chemistry, 15.06.2021 14:00 msladycie8831
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antifreeze, and plastics. Glycerol is a water-soluble liquid
with a density of 1.2656 g/mL at 15 °C. Calculate the molarity of a solution of glycerol made by dissolving 50.000 mL glycerol at 15 °C in enough water to
make 250.00 mL of solution. The molecular weight of C3HgO3 is 92.094 amu.
O A 0.6871
O B. 3.600
O C. 63.28
O 0.92.10
O E. 2.749

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Glycerol. C3HgO3, is a substance used extensively in the manufacture of cosmetics, foodstuffs, antif...
Questions


Geography, 02.12.2020 18:40


Biology, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40

English, 02.12.2020 18:40


Chemistry, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40



Mathematics, 02.12.2020 18:40

Chemistry, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40

Spanish, 02.12.2020 18:40

Biology, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40


Advanced Placement (AP), 02.12.2020 18:40

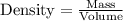 ......(1)
......(1)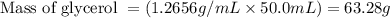
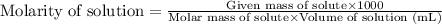 .....(2)
.....(2)