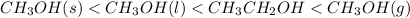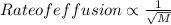CH3OH(l),


Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 14:30
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1

Chemistry, 23.06.2019 18:30
Esure to answer all parts. the equilibrium constant for the reaction ni2+(aq) + 6 nh3(aq) ⇌ ni(nh3)6 2+(aq) is kf = 5.6 × 108 at 25°c. (a) what is δg o at this temperature? (b) if standard-state concentrations of reactants and products are mixed, in which direction does the reaction proceed? (c) determine δg when [ni(nh3)62+] = 0.010 m, [ni2+] = 0.0010 m, and [nh3] = 0.0050 m. in which direction will the reaction proceed to achieve equilibrium? (a) × 10 j/mol (enter your answer in scientific notation.) (b) to the right. to the left. (c) × 10 j/mol (enter your answer in scientific notation.) to the right. to the left.
Answers: 3

Chemistry, 24.06.2019 00:00
What did ernest rutherford’s gold foil experiment demonstrate about atoms?
Answers: 1
You know the right answer?
Arrange the compounds in order of increasing entropy (S).
CH3OH(s)
CH3OH(l),
CH3OH(l),
Questions


Mathematics, 14.04.2021 01:00


Mathematics, 14.04.2021 01:00


Mathematics, 14.04.2021 01:00





Mathematics, 14.04.2021 01:00

English, 14.04.2021 01:00

Mathematics, 14.04.2021 01:00

Biology, 14.04.2021 01:00


Advanced Placement (AP), 14.04.2021 01:00

Mathematics, 14.04.2021 01:00

History, 14.04.2021 01:00

Geography, 14.04.2021 01:00

Arts, 14.04.2021 01:00