Name the following carboxylic
acid:
OH
O
A. butanoic acid
B. butanol
...

Chemistry, 14.06.2021 23:50 dakotalynnwillis01
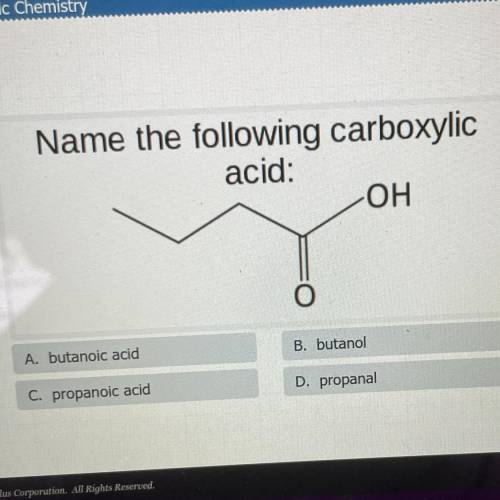
Name the following carboxylic
acid:
OH
O
A. butanoic acid
B. butanol
C. propanoic acid
D. propanal

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Questions


World Languages, 01.06.2021 14:00

Mathematics, 01.06.2021 14:00


Computers and Technology, 01.06.2021 14:00




Spanish, 01.06.2021 14:00

Mathematics, 01.06.2021 14:00

Mathematics, 01.06.2021 14:00

Computers and Technology, 01.06.2021 14:00


Mathematics, 01.06.2021 14:00

Mathematics, 01.06.2021 14:00

Mathematics, 01.06.2021 14:00

English, 01.06.2021 14:00

Physics, 01.06.2021 14:00


History, 01.06.2021 14:00



