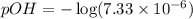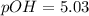Chemistry, 14.06.2021 15:20 galaxychild101
A solution of acetic acid that has a concentration of 0.10 moles per liter has a pH of 2.87. What is the likely pH of a 0.10 mole per liter solution of the conjugate base sodium acetate?
A. 8.97
B. 1.00
C. 2.87
D. 4.74
E. 13.00

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
A solution of acetic acid that has a concentration of 0.10 moles per liter has a pH of 2.87. What is...
Questions

Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30

Chemistry, 18.12.2020 01:30


English, 18.12.2020 01:30






Mathematics, 18.12.2020 01:30


Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30

Social Studies, 18.12.2020 01:30


Arts, 18.12.2020 01:30

Arts, 18.12.2020 01:30

History, 18.12.2020 01:30

Chemistry, 18.12.2020 01:30

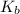 of a reaction, we use the equation:
of a reaction, we use the equation: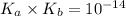
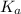 = acid dissociation constant of acetic acid =
= acid dissociation constant of acetic acid = 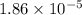
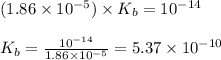
![[OH^-]=\sqrt{K_b\times \text{[Conjugate base]}}](/tpl/images/1373/6739/be895.png)
![[OH^-]=\sqrt{(5.37\times 10^{-10})\times 0.1}](/tpl/images/1373/6739/6c6fd.png)
![[OH^-]=7.33\times 10^{-6}](/tpl/images/1373/6739/66957.png)
![pOH=-\log [OH^-]](/tpl/images/1373/6739/1fac1.png)