
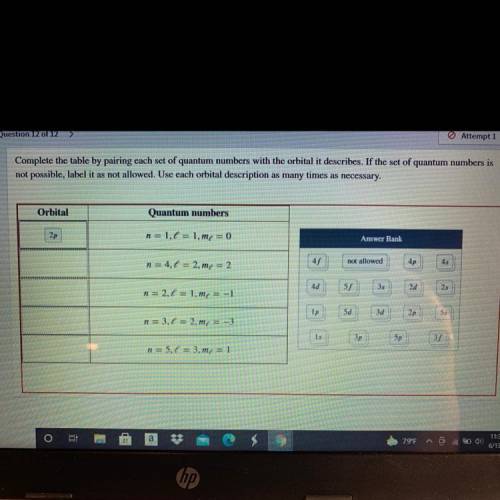
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set of quantum numbers is
not possible, label it as not allowed. Use each orbital description as many times as necessary.
Orbital
Quantum numbers
2p
n = 1.6 = 1.me = 0
Answer Bank
n = 4.1 = 2.m = 2
45
not allowed
4p
45
4d
n = 2.1 = 1.mx = -1
55
35
2d
25
Ip
5d
3d
2p
55
n = 3,6 = 2.mx = -3
1s
3p
5p
35
n = 5.0 = 3.m4 = 1
7
O
Bi
11:36 PM

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Complete the table by pairing each set of quantum numbers with the orbital it describes. If the set...
Questions

English, 23.12.2019 07:31


Health, 23.12.2019 07:31




Mathematics, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31


English, 23.12.2019 07:31


Biology, 23.12.2019 07:31



History, 23.12.2019 07:31


History, 23.12.2019 07:31



History, 23.12.2019 07:31



