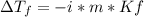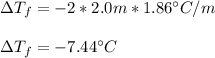Chemistry, 13.06.2021 14:00 VgCarlos3787
What is the expected freezing-point depression for a solution that contains 2.0 mol of KCI
(electrolyte) dissolved in 1.0 kg of water? (K,- -1.86°C/m.)
a.-7.44 °C
C. +7.44 °C
b.-4.77 °C
d. +4.7 °C

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
What is the expected freezing-point depression for a solution that contains 2.0 mol of KCI
(electro...
Questions



Mathematics, 03.09.2021 16:30

Computers and Technology, 03.09.2021 16:30


Mathematics, 03.09.2021 16:30


English, 03.09.2021 16:30

Mathematics, 03.09.2021 16:30

Social Studies, 03.09.2021 16:30

Business, 03.09.2021 16:30


History, 03.09.2021 16:30


Computers and Technology, 03.09.2021 16:30

Business, 03.09.2021 16:30

Mathematics, 03.09.2021 16:30



Computers and Technology, 03.09.2021 16:30