
Chemistry, 13.06.2021 09:20 dylanentwistle5197
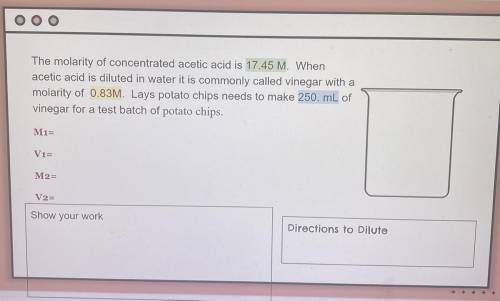
The molarity of concentrated acetic acid is 17.45 M. When acetic acid is diluted in water it is commonly called vinegar with a molarity of 0.83M. Lays potato chips needs to make 250. mL of vinegar for a test batch of potato chips.
V1=
M1=
V2=
M2=

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
The molarity of concentrated acetic acid is 17.45 M. When acetic acid is diluted in water it is comm...
Questions

Mathematics, 11.02.2020 11:59


Mathematics, 11.02.2020 12:16

Mathematics, 11.02.2020 12:16

Mathematics, 11.02.2020 12:16

Spanish, 11.02.2020 12:16

Social Studies, 11.02.2020 12:17

Mathematics, 11.02.2020 12:18


Mathematics, 11.02.2020 12:18



Mathematics, 11.02.2020 12:18




Mathematics, 11.02.2020 12:38


Mathematics, 11.02.2020 12:38



