
Chemistry, 12.06.2021 01:40 benjaminmccutch
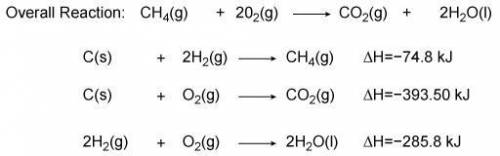
Use the information from the diagram to calculate the enthalpy of combustion for methane.
Question 2 options:
A)
+752 kJ
B)
–921 kJ
C)
–604 kJ
D)
+604 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Use the information from the diagram to calculate the enthalpy of combustion for methane.
Question...
Questions

Mathematics, 14.06.2021 04:30

Chemistry, 14.06.2021 04:30


Biology, 14.06.2021 04:30

History, 14.06.2021 04:30

Mathematics, 14.06.2021 04:30

Mathematics, 14.06.2021 04:30

English, 14.06.2021 04:30

Mathematics, 14.06.2021 04:30




Social Studies, 14.06.2021 04:30



Business, 14.06.2021 04:30

History, 14.06.2021 04:30


Physics, 14.06.2021 04:30




