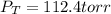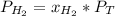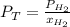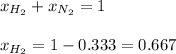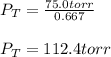Chemistry, 11.06.2021 23:10 ghwolf4p0m7x0
In a mixture of hydrogen and nitrogen gases, the mole fraction of nitrogen is 0.333. If the partial pressure of hydrogen in the mixture is 75.0 torr, what is the total pressure of the mixture

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1


Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
In a mixture of hydrogen and nitrogen gases, the mole fraction of nitrogen is 0.333. If the partial...
Questions


English, 05.05.2021 23:50





History, 05.05.2021 23:50


Mathematics, 05.05.2021 23:50

Mathematics, 05.05.2021 23:50

Social Studies, 05.05.2021 23:50



Engineering, 05.05.2021 23:50

History, 05.05.2021 23:50



Biology, 05.05.2021 23:50

Health, 05.05.2021 23:50