Chemistry, 11.06.2021 22:50 tyneshiajones124
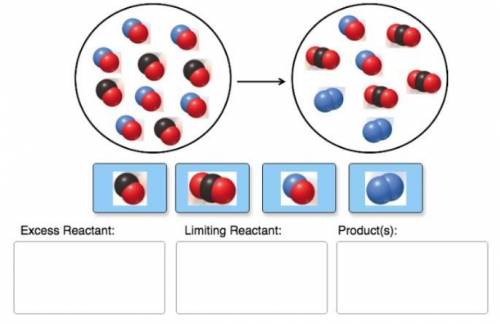
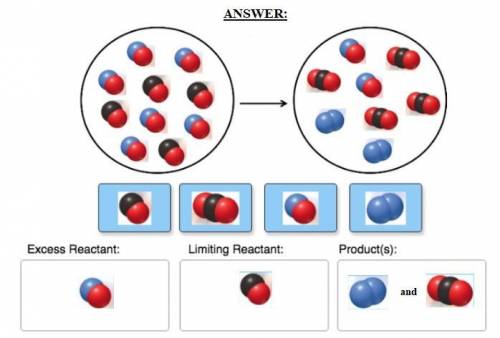
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g) + 2 CO (g) → N2 (g) + 2 CO2 (g) Identify the excess reactant, the limiting reactant, and the product(s) using the molecular art. (Black spheres are carbon, blue spheres are nitrogen, and red spheres are oxygen.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

You know the right answer?
Consider the reaction of NO and CO to form N2 and CO2, according to the balanced equation: 2 NO (g)...
Questions






Mathematics, 01.03.2021 01:00


History, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00


History, 01.03.2021 01:00

Biology, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00

English, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00

Chemistry, 01.03.2021 01:00

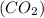 compound
compound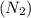
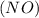 compound
compound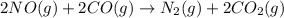
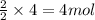 of NO
of NO