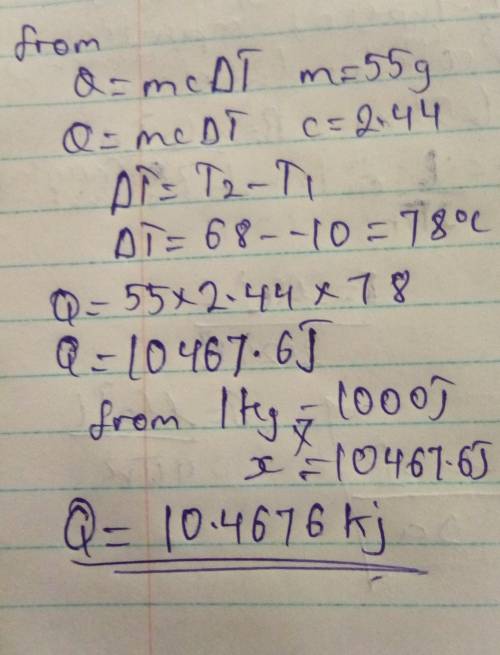Chemistry, 11.06.2021 14:00 kennethDA19
The specific heat of ethanol is 2.44 J/(g °C). How many kilojoules of energy are required to heat 55.0 g of ethanol from -10.0°C to 68.0°C

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
The specific heat of ethanol is 2.44 J/(g °C). How many kilojoules of energy are required to heat 55...
Questions

Biology, 27.06.2021 07:00




Law, 27.06.2021 07:00

Biology, 27.06.2021 07:00


Mathematics, 27.06.2021 07:00

Physics, 27.06.2021 07:00



English, 27.06.2021 07:00

Computers and Technology, 27.06.2021 07:00


Mathematics, 27.06.2021 07:00

Mathematics, 27.06.2021 07:00

Computers and Technology, 27.06.2021 07:00


History, 27.06.2021 07:00