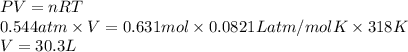Chemistry, 11.06.2021 06:50 bgallman153p71edg
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume of the container?
R = 0.0821 L atm/mol K
15.1 L
4.29 L
30.3 L
2.14 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume...
Questions


Mathematics, 03.02.2020 22:52

Mathematics, 03.02.2020 22:52

History, 03.02.2020 22:52


History, 03.02.2020 22:52

Mathematics, 03.02.2020 22:52

Mathematics, 03.02.2020 22:52


Spanish, 03.02.2020 22:52


Mathematics, 03.02.2020 22:52




Mathematics, 03.02.2020 22:52

Mathematics, 03.02.2020 22:52

English, 03.02.2020 22:52


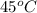 = (45 + 273) K = 318 K
= (45 + 273) K = 318 K