
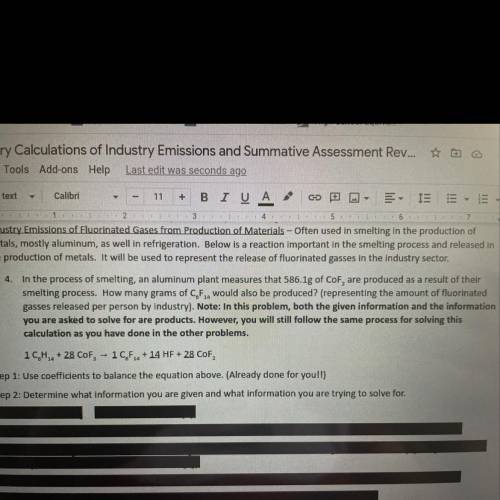
Chemistry, 11.06.2021 04:30 blessingjessica
In the process of smelting, an aluminum plant measures that 586.1g of CoF2, are produced as a result of their
smelting process. How many grams of C6F14 would also be produced? (representing the amount of fluorinated
gasses released per person by Industry). Note: In this problem, both the given information and the information
you are asked to solve for are products. However, you will still follow the same process for solving this
calculation as you have done in the other problems.
1 C6H14 + 28 CoF3, 1C6F14 + 14 HF + 28 CoF2.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
In the process of smelting, an aluminum plant measures that 586.1g of CoF2, are produced as a result...
Questions





Computers and Technology, 27.04.2021 15:20








Mathematics, 27.04.2021 15:20






History, 27.04.2021 15:20



