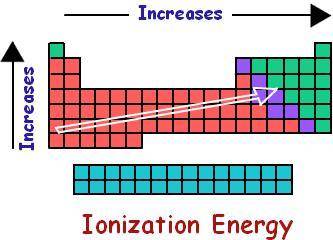
The first ionization energy of an element is the energy required to remove an electron from a gaseous atom of an element to produce a +1 ion:
M(g) + energy ---> M ^(+) (g) + e -
How do you think the activity of an element ought to be related to its first ionization energy? Predict a decreasing order of reactivity of the above elements based on their first ionization energies.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
The first ionization energy of an element is the energy required to remove an electron from a gaseou...
Questions



Mathematics, 13.06.2020 23:57





Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57

Social Studies, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57



Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57