
Chemistry, 11.06.2021 02:00 alydiale584
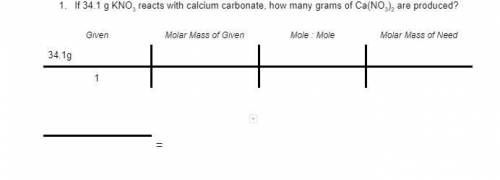
2KNO3 + CaCO3 → K2CO3 + Ca(NO3)2
If 34.1 g KNO3 reacts with calcium carbonate, how many grams of Ca(NO3)2 are produced?
1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
2KNO3 + CaCO3 → K2CO3 + Ca(NO3)2
If 34.1 g KNO3 reacts with calcium carbonate, how many grams of Ca...
Questions

Chemistry, 24.02.2021 07:50

Mathematics, 24.02.2021 07:50









Chemistry, 24.02.2021 07:50


Mathematics, 24.02.2021 07:50

Health, 24.02.2021 07:50

Mathematics, 24.02.2021 07:50


Mathematics, 24.02.2021 07:50

Arts, 24.02.2021 07:50

History, 24.02.2021 07:50

History, 24.02.2021 07:50



