Question 17 of 30
A mixture of He, Ne, and N, gases has a pressure of 1.943 atm. If the
press...

Chemistry, 10.06.2021 19:30 babyambs50
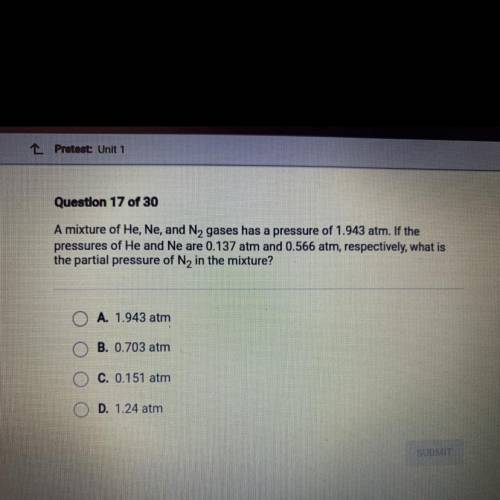
Question 17 of 30
A mixture of He, Ne, and N, gases has a pressure of 1.943 atm. If the
pressures of He and Ne are 0.137 atm and 0.566 atm, respectively, what is
the partial pressure of N2 in the mixture?
A. 1.943 atm
B. 0.703 atm
C. 0.151 atm
D. 1.24 atm
SUBMIT

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
Questions

History, 19.03.2020 01:36


Mathematics, 19.03.2020 01:36

Chemistry, 19.03.2020 01:36

Mathematics, 19.03.2020 01:36

Mathematics, 19.03.2020 01:36



Geography, 19.03.2020 01:36



Biology, 19.03.2020 01:36

History, 19.03.2020 01:36



History, 19.03.2020 01:36

Chemistry, 19.03.2020 01:36

Mathematics, 19.03.2020 01:36

Health, 19.03.2020 01:36

Mathematics, 19.03.2020 01:36



