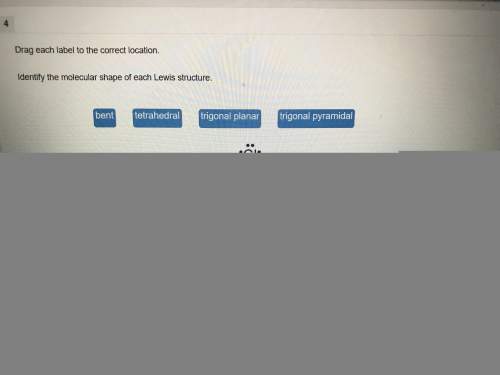
A solution of ammonia NH3(aq) is at equilibrium. How would the equilibrium
change if OH were added to the solution?
A. [NH4+) and (H*] would decrease.
B. [NH4+) and (OH] would increase.
C. (NH4+) and (OH] would decrease.
D. (NH4+] would increase and [OH-] would decrease

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
A solution of ammonia NH3(aq) is at equilibrium. How would the equilibrium
change if OH were added...
Questions


Health, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


English, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



Physics, 12.12.2020 15:50

English, 12.12.2020 15:50



English, 12.12.2020 15:50



Geography, 12.12.2020 15:50



Physics, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50