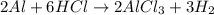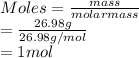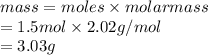Chemistry, 10.06.2021 15:20 drandbone92
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid according to the following balanced chemical equation: 2 Al + 6 HCl → 2 AlCl3 + 3 H2

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
Calculate the mass of hydrogen formed when 26.98 g of aluminum reacts with excess hydrochloric acid...
Questions





Mathematics, 24.11.2020 20:20



Medicine, 24.11.2020 20:20


History, 24.11.2020 20:20

Health, 24.11.2020 20:20

Mathematics, 24.11.2020 20:20

Mathematics, 24.11.2020 20:20

Biology, 24.11.2020 20:20



English, 24.11.2020 20:20

World Languages, 24.11.2020 20:20

English, 24.11.2020 20:20

Mathematics, 24.11.2020 20:20