
Chemistry, 09.06.2021 09:50 aliceohern
A solution containing 1.22 g of a diprotic acid H2CH2O4 (malonic acid)
was titrated with 45.5 mL of NaOH to reach the second equivalence
point. What is the concentration of the NaOH solution? (MW_malonic
acid = 104.06 g/mol)

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
A solution containing 1.22 g of a diprotic acid H2CH2O4 (malonic acid)
was titrated with 45.5 mL of...
Questions

Mathematics, 19.05.2020 03:26

Business, 19.05.2020 03:26

English, 19.05.2020 03:26




Mathematics, 19.05.2020 03:26

Mathematics, 19.05.2020 03:26


History, 19.05.2020 03:26


English, 19.05.2020 03:26

Mathematics, 19.05.2020 03:26


English, 19.05.2020 03:26




English, 19.05.2020 03:26

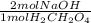 = 0.02344 mol NaOH
= 0.02344 mol NaOH

