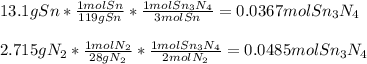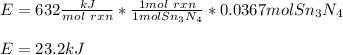How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N...

Chemistry, 09.06.2021 07:50 matthewlucas8613
How much energy is produced when 13.1 g of tin reacts with 2.715 g of N2 ?
I.
3 Sn + 2 N2Sn3N4 + 632 KJ
Hint change grams to moles first.
1 mole Sn= 119g
1 mole N2= 28 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1


Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Questions






Business, 06.05.2021 14:00

Health, 06.05.2021 14:00


Mathematics, 06.05.2021 14:00

Mathematics, 06.05.2021 14:00

Business, 06.05.2021 14:00

Geography, 06.05.2021 14:00

Biology, 06.05.2021 14:00

Mathematics, 06.05.2021 14:00





Biology, 06.05.2021 14:00

Business, 06.05.2021 14:00