
Chemistry, 09.06.2021 03:40 autumnkiewel200
Which of the following elements/compounds are being oxidized in the following equation?
A) Manganese
B) Oxygen
C) Zinc
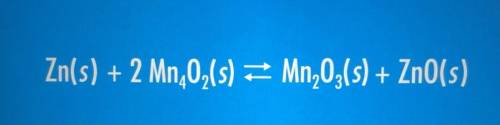

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
Which of the following elements/compounds are being oxidized in the following equation?
A) Manganes...
Questions

Social Studies, 26.01.2020 00:31


Mathematics, 26.01.2020 00:31






Mathematics, 26.01.2020 00:31

World Languages, 26.01.2020 00:31



Mathematics, 26.01.2020 00:31



History, 26.01.2020 00:31

History, 26.01.2020 00:31


Social Studies, 26.01.2020 00:31

Mathematics, 26.01.2020 00:31



