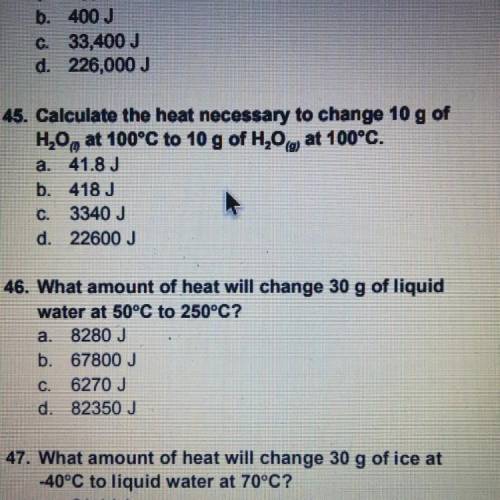Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Calculate the heat necessary to change 10 g of H20, at 100°C to 10 g of H20 at 100°C.
a. 41.8 J
Questions

Biology, 21.07.2019 13:10





History, 21.07.2019 13:10


Biology, 21.07.2019 13:10

Mathematics, 21.07.2019 13:10

History, 21.07.2019 13:10


Mathematics, 21.07.2019 13:10





Social Studies, 21.07.2019 13:10



Mathematics, 21.07.2019 13:10