Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
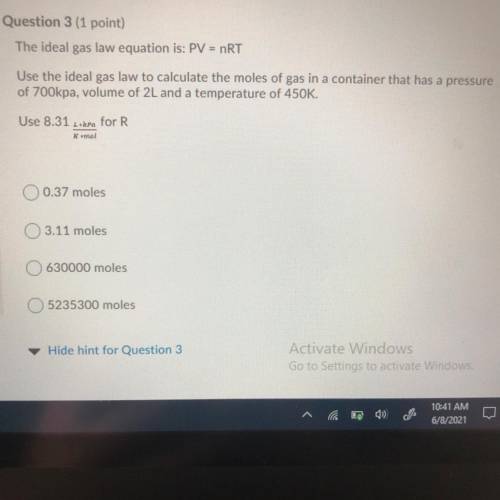
The ideal gas law equation is: PV = nRT
Use the ideal gas law to calculate the moles of gas in a co...
Questions

Social Studies, 09.10.2019 14:10

History, 09.10.2019 14:10



Chemistry, 09.10.2019 14:10

Mathematics, 09.10.2019 14:10


Mathematics, 09.10.2019 14:10



Biology, 09.10.2019 14:10

Biology, 09.10.2019 14:10


Geography, 09.10.2019 14:10


Chemistry, 09.10.2019 14:10

Geography, 09.10.2019 14:10

History, 09.10.2019 14:10

Physics, 09.10.2019 14:10