
Chemistry, 08.06.2021 08:00 emilaw3233
The reaction of bromine gas with chlorine gas, shown here, has a Keq value of 7.20 at 200°C. If a closed vessel was charged with the two reactants, each at an initial concentration of 0.200 M, but with no initial concentration of BrCl, what would be the equilibrium concentration of Br2, Cl2 and BrCl(g)? Br2(g) + Cl2(g) ↔ 2BrCl(g) K = 7.20

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
The reaction of bromine gas with chlorine gas, shown here, has a Keq value of 7.20 at 200°C. If a cl...
Questions








Computers and Technology, 01.08.2021 20:50


Biology, 01.08.2021 20:50




Mathematics, 01.08.2021 21:00




Mathematics, 01.08.2021 21:00


English, 01.08.2021 21:00

![[Cl_2]=[Br_2]=0.856M](/tpl/images/1366/5138/5290e.png)
![[BrCl]=0.229M](/tpl/images/1366/5138/704d0.png)
![Keq=\frac{[BrCl]^2}{[Cl_2][Br_2]}](/tpl/images/1366/5138/7a521.png)
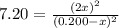
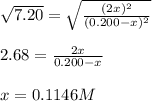
![[Cl_2]=[Br_2]=0.200M-0.1146M=0.856M](/tpl/images/1366/5138/7f591.png)
![[BrCl]=2*0.1146M=0.229M](/tpl/images/1366/5138/64b19.png)


