
Chemistry, 08.06.2021 03:20 avisconti571
A sample of 0.2140 g of an unkown substance monoprotic acid was dissolved in 25.0 mL of water and titrated with 0.950 M NaOH. The acid required 27.4 mL of base to reach the equivalence point. After 15.0 mL of base had been added in the titration, the pH was found to be 6.50. What is the Ka for the unknown acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
A sample of 0.2140 g of an unkown substance monoprotic acid was dissolved in 25.0 mL of water and ti...
Questions

Biology, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00


Mathematics, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00

English, 17.10.2019 19:00

Social Studies, 17.10.2019 19:00

Health, 17.10.2019 19:00


Mathematics, 17.10.2019 19:00


English, 17.10.2019 19:00




Mathematics, 17.10.2019 19:00



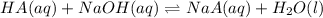
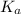 of HA
of HA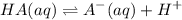
![K_a=\frac{[A^-].[H^+]}{[HA]}](/tpl/images/1366/2932/3c83d.png)
![$[HA] = \frac{^nH_A}{V}$](/tpl/images/1366/2932/b0ec6.png)
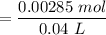
![$[NaOH]= \frac{0.015L \times 0.0950 M}{V}$](/tpl/images/1366/2932/a5013.png)
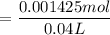

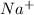 and 0.0356 M
and 0.0356 M 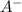
![[H^+]](/tpl/images/1366/2932/07acb.png)
![$[H^+] = 10^{-pH}$](/tpl/images/1366/2932/d6eff.png)
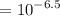
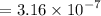
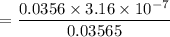
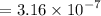
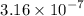 .
.

