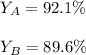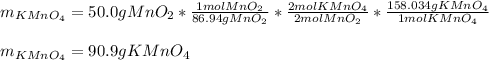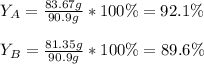Scientist A produces 83.67 g KMnO4 while Scientist B produces 81.35 g KMnO4.
What is the percent yield for Scientist A?
What is the percent yield for Scientist B?
You must show all work to receive full credit.
The equation for the production of potassium permanganate is as follows:
2 MnO2 + 2 KOH + O2 → 2 KMnO4 + H2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Scientist A produces 83.67 g KMnO4 while Scientist B produces 81.35 g KMnO4.
What is the percent yi...
Questions

History, 12.04.2021 14:30


English, 12.04.2021 14:30


Physics, 12.04.2021 14:30

Biology, 12.04.2021 14:30

Mathematics, 12.04.2021 14:30

Computers and Technology, 12.04.2021 14:30


English, 12.04.2021 14:30

Physics, 12.04.2021 14:30

Mathematics, 12.04.2021 14:30





History, 12.04.2021 14:30


Mathematics, 12.04.2021 14:40

Social Studies, 12.04.2021 14:40