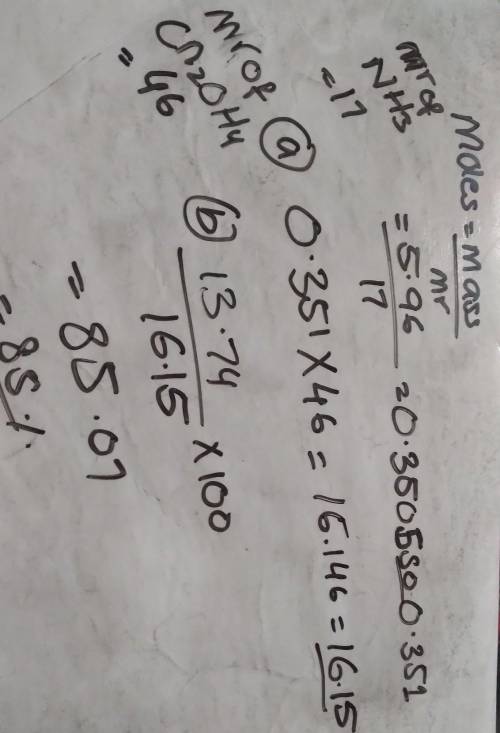Chemistry, 05.06.2021 22:40 GreenHerbz206
5.96 g of ammonia reacts completely according to the following reaction:
2 NH, (g) + Co, (g) → CN, OH, (s) + H20 (1)
(a) What is the theoretical yield of urea (CN, OH,) for this reaction?
(b) If 13.74 g of urea are produced, what is the percent yield for this equation?
please show work, will give brainliest

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

You know the right answer?
5.96 g of ammonia reacts completely according to the following reaction:
2 NH, (g) + Co, (g) → CN,...
Questions

Mathematics, 26.08.2019 17:20




Computers and Technology, 26.08.2019 17:20






Computers and Technology, 26.08.2019 17:20






Computers and Technology, 26.08.2019 17:20

Computers and Technology, 26.08.2019 17:20

Biology, 26.08.2019 17:30