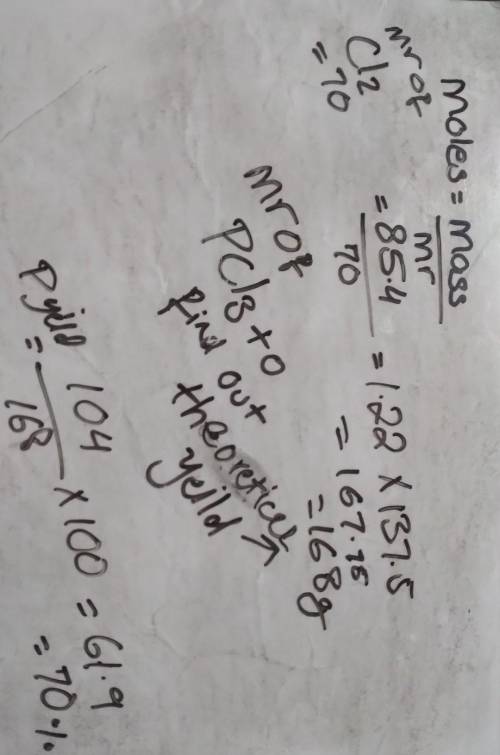Chemistry, 05.06.2021 22:40 Angeldelissa
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13 (I)
If 104 g of phosphorous trichloride is produced, what is the percent yield for this reaction?
please show work
will give brainliest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 08:30
Plz a person walks 1 mile every day for exercise, leaving her front porch at 9 am and returning to her front porch at 9: 25 am what was the total displacement of her daily walk a. 1 mile b. 0 c. 25 min d. none of the above
Answers: 2
You know the right answer?
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13...
Questions


Physics, 05.01.2021 15:00

English, 05.01.2021 15:00

Mathematics, 05.01.2021 15:00

Chemistry, 05.01.2021 15:00

Mathematics, 05.01.2021 15:00

Mathematics, 05.01.2021 15:00


Mathematics, 05.01.2021 15:00


Mathematics, 05.01.2021 15:00

Geography, 05.01.2021 15:00



Mathematics, 05.01.2021 15:00


Mathematics, 05.01.2021 15:00

English, 05.01.2021 15:00

English, 05.01.2021 15:00

Spanish, 05.01.2021 15:00