
Chemistry, 04.06.2021 23:20 alex12everett
A monoprotic weak acid when dissolved in water is 0.66% dissociated and produces a solution with a pH of 3.04. Calculate the Ka of the acid. g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
A monoprotic weak acid when dissolved in water is 0.66% dissociated and produces a solution with a p...
Questions

Mathematics, 28.08.2019 14:50

History, 28.08.2019 14:50

Biology, 28.08.2019 14:50


English, 28.08.2019 14:50


Health, 28.08.2019 14:50

Biology, 28.08.2019 14:50


Biology, 28.08.2019 14:50






Mathematics, 28.08.2019 14:50

Mathematics, 28.08.2019 14:50

Mathematics, 28.08.2019 14:50


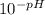 [H⁺] = 9.12x10⁻⁴ M
[H⁺] = 9.12x10⁻⁴ M![\frac{[9.12x10^{-4}]*[9.12x10^{-4}]}{[0.138]}](/tpl/images/1363/4628/7f320.png) = 6.02x10⁻⁶
= 6.02x10⁻⁶

