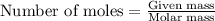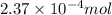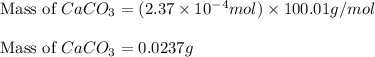Chemistry, 04.06.2021 20:20 jujulakaeuaws
Calcium carbonate is often used as an antacid. Your stomach acid is composed of HCl at a pH of 1.5. If you ate t much Turkey and need to neutralize 15.0 mL of stomach acid, how many grams of calcium carbonate would you need to take

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2


Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Calcium carbonate is often used as an antacid. Your stomach acid is composed of HCl at a pH of 1.5....
Questions







Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00

English, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00



Social Studies, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00



![pH=-\log [H^+]](/tpl/images/1363/1507/37e81.png) .....(1)
.....(1)![1.5=-\log[H^+]](/tpl/images/1363/1507/d7b07.png)
![[H^+]=10^{(-1.5)}=0.0316M](/tpl/images/1363/1507/dca45.png)
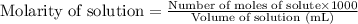 .....(2)
.....(2)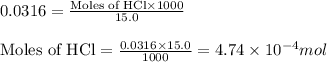
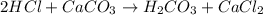
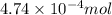 of HCl will react with =
of HCl will react with = 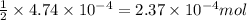 of calcium carbonate
of calcium carbonate