
Chemistry, 04.06.2021 18:00 gissellesolorza
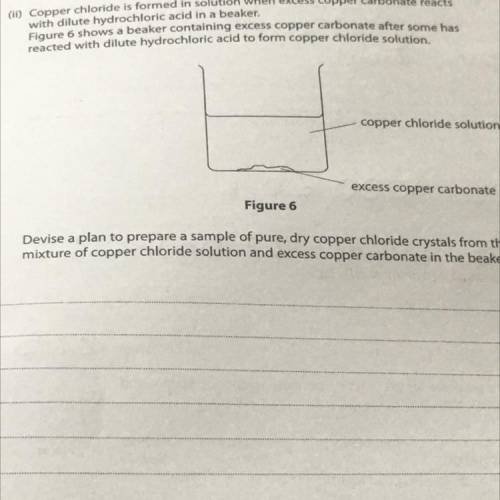
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochloric acid in a beaker.
Figure 6 shows a beaker containing excess copper carbonate after some has
reacted with dilute hydrochloric acid to form copper chloride solution.
DO NOT WRITE IN THIS AREA
DO NOT WRITE IN THIS AREN
copper chloride solution
excess copper carbonate
Figure 6
Devise a plan to prepare a sample of pure, dry copper chloride crystals from the
mixture of copper chloride solution and excess copper carbonate in the beaker.
DO NOT
THIS AREA
A

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochl...
Questions




Mathematics, 19.06.2020 03:57

Mathematics, 19.06.2020 03:57



English, 19.06.2020 03:57



Mathematics, 19.06.2020 03:57




Mathematics, 19.06.2020 03:57



Mathematics, 19.06.2020 04:57




