
Chemistry, 04.06.2021 03:50 toribrown3773
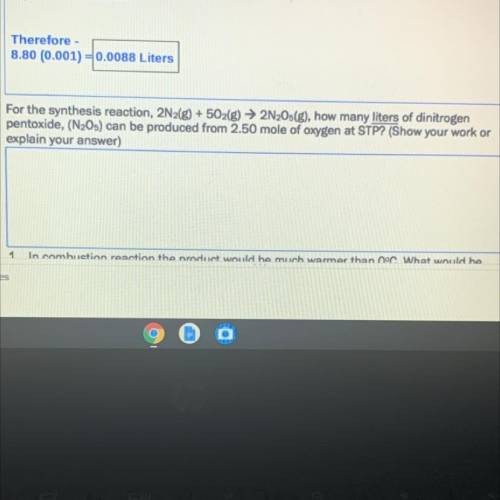
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N205) can be produced from 2.50 mole of oxygen at STP? (Show your work or
explain your answer)
*SEE PICTURE FOR BETTER UNDERSTANDING*

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
For the synthesis reaction, 2N2(g) + 5O2(g) → 2N2Os(g), how many liters of dinitrogen
pentoxide, (N...
Questions

Chemistry, 15.04.2020 15:35


Arts, 15.04.2020 15:35











Social Studies, 15.04.2020 15:35

Business, 15.04.2020 15:35

Computers and Technology, 15.04.2020 15:35






