Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
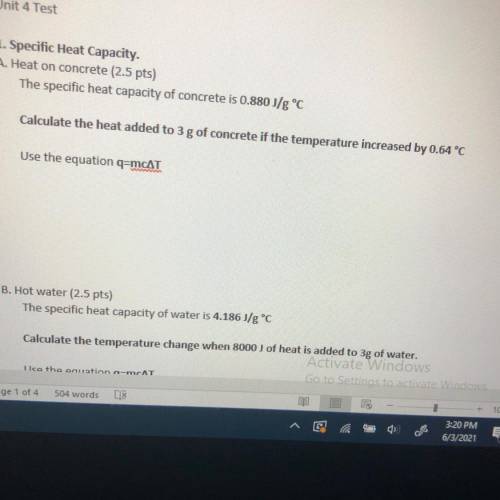
The specific heat capacity of concrete is 0.880 J/g °C
Calculate the heat added to 3 g of concrete...
Questions


Mathematics, 11.03.2021 01:00



Mathematics, 11.03.2021 01:00

Mathematics, 11.03.2021 01:00

Biology, 11.03.2021 01:00

Chemistry, 11.03.2021 01:00


Spanish, 11.03.2021 01:00





Mathematics, 11.03.2021 01:00



Mathematics, 11.03.2021 01:00

Chemistry, 11.03.2021 01:00