
Chemistry, 03.06.2021 18:00 hjeffrey168
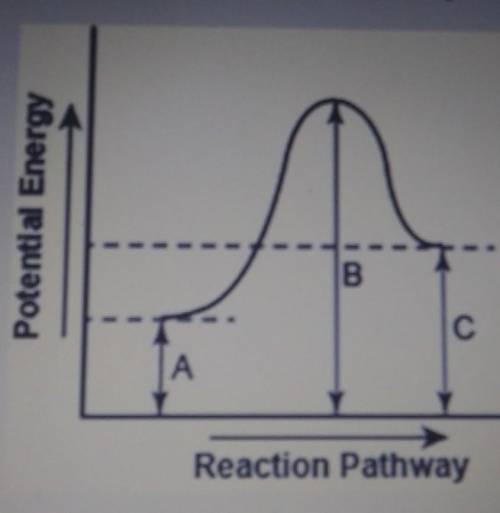
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagram illustrate an enothermic or an exothermic reaction? Give reasons to support your answer.
Part 2: Describe how you can determine the total change in ethalpy and activation energy from the diagram and if each is positive or negative.
Brainliest if you give the definition of endo and exothermic reactions, and how you know the change in enthalpy even if you put nothing else.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2
You know the right answer?
The diagram below shows the potential energy changes for a reaction pathway.
Part 1: Does the diagr...
Questions

History, 05.09.2020 08:01


World Languages, 05.09.2020 08:01

Mathematics, 05.09.2020 08:01


Mathematics, 05.09.2020 08:01


Chemistry, 05.09.2020 08:01


Mathematics, 05.09.2020 08:01


Chemistry, 05.09.2020 08:01


English, 05.09.2020 08:01



Mathematics, 05.09.2020 08:01

English, 05.09.2020 08:01


Computers and Technology, 05.09.2020 08:01



