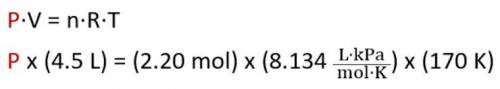Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

You know the right answer?
What is the pressure of 2.20 mol of a gas stored in a 4.5 L container at a temperature of 170 K? (Us...
Questions




Geography, 22.08.2020 17:01

History, 22.08.2020 17:01

Mathematics, 22.08.2020 17:01


Mathematics, 22.08.2020 17:01


History, 22.08.2020 17:01







Mathematics, 22.08.2020 17:01

History, 22.08.2020 17:01

Mathematics, 22.08.2020 17:01