
Chemistry, 03.06.2021 01:50 KnMcdonaldk93906
Now we need to find the amount of NF3 that can be formed by the complete reactions of each of the reactants. If all of the N2 was used up in the reaction, how many moles of NF3 would be produced

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 23.06.2019 11:40
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
You know the right answer?
Now we need to find the amount of NF3 that can be formed by the complete reactions of each of the re...
Questions

Health, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

English, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

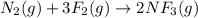
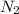 is reacted with 19.3 g of
is reacted with 19.3 g of 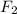 . Now we need to find the amount of
. Now we need to find the amount of 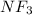 that can be formed by the complete reactions of each of the reactants.
that can be formed by the complete reactions of each of the reactants.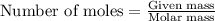 ......(1)
......(1)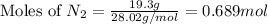
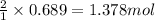 of
of 

