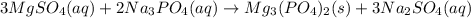Chemistry, 03.06.2021 01:00 justaguy15
When an aqueous solution of sodium phosphate (Na3PO4) and an aqueous solution of magnesium sulfate (MgSO4) are mixed, a precipitate forms. Write the chemical formula of the precipitate.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2

Chemistry, 23.06.2019 15:00
Use the periodic table to identify the number of core electrons and the number of valence electrons in each case below. potassium (k): 1s22s22p63s23p64s1
Answers: 1

Chemistry, 23.06.2019 16:10
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2
You know the right answer?
When an aqueous solution of sodium phosphate (Na3PO4) and an aqueous solution of magnesium sulfate (...
Questions


Mathematics, 31.08.2020 15:01

English, 31.08.2020 15:01

History, 31.08.2020 16:01


English, 31.08.2020 16:01



Spanish, 31.08.2020 16:01

Mathematics, 31.08.2020 16:01

Social Studies, 31.08.2020 16:01

Social Studies, 31.08.2020 16:01

Mathematics, 31.08.2020 16:01

Mathematics, 31.08.2020 16:01

Mathematics, 31.08.2020 16:01

English, 31.08.2020 16:01

Social Studies, 31.08.2020 16:01


Mathematics, 31.08.2020 16:01

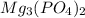 .
.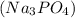 and an aqueous solution of magnesium sulfate
and an aqueous solution of magnesium sulfate 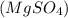 are mixed, a precipitate forms is as follows.
are mixed, a precipitate forms is as follows.