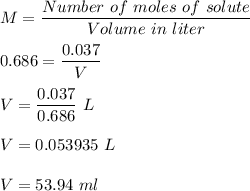
Chemistry, 02.06.2021 04:40 triplejjj964
What volume of 0.686 M HCl would contain 0.037 moles of solute?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
What volume of 0.686 M HCl would contain 0.037 moles of solute?...
Questions

Biology, 17.01.2020 01:31


Mathematics, 17.01.2020 01:31

Mathematics, 17.01.2020 01:31

Mathematics, 17.01.2020 01:31

Mathematics, 17.01.2020 01:31



Chemistry, 17.01.2020 01:31

Social Studies, 17.01.2020 01:31




Mathematics, 17.01.2020 01:31


Mathematics, 17.01.2020 01:31


Mathematics, 17.01.2020 01:31


Mathematics, 17.01.2020 01:31