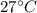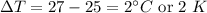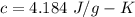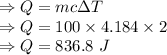Chemistry, 02.06.2021 01:00 GreenHerbz206
A hot metal is placed in 100.0 g sample of water and it causes the temperature of the water to increase from 25 C to 27 C. How many joules of energy did the water absorb from the metal?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
A hot metal is placed in 100.0 g sample of water and it causes the temperature of the water to incre...
Questions


Biology, 19.05.2020 16:07


Mathematics, 19.05.2020 16:07

Mathematics, 19.05.2020 16:07


Social Studies, 19.05.2020 16:07


Mathematics, 19.05.2020 16:07



Physics, 19.05.2020 16:07


Mathematics, 19.05.2020 16:07

Mathematics, 19.05.2020 16:07



Mathematics, 19.05.2020 16:07

Mathematics, 19.05.2020 16:07

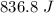
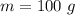
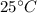 to
to