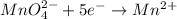Which of these half-reactions represents reduction?
I. Fe2+ → Fe3+
II. Cr2O72- → Cr3+
I...

Chemistry, 01.06.2021 21:50 maytce7237
Which of these half-reactions represents reduction?
I. Fe2+ → Fe3+
II. Cr2O72- → Cr3+
III. MnO4- → Mn2+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Questions

Biology, 25.07.2019 00:00


Mathematics, 25.07.2019 00:00

Social Studies, 25.07.2019 00:00




Business, 25.07.2019 00:00




Advanced Placement (AP), 25.07.2019 00:00

Biology, 25.07.2019 00:00

Geography, 25.07.2019 00:00


Mathematics, 25.07.2019 00:00



Geography, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00

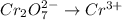
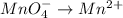
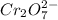 is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.
is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.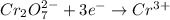
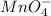 is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.
is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.