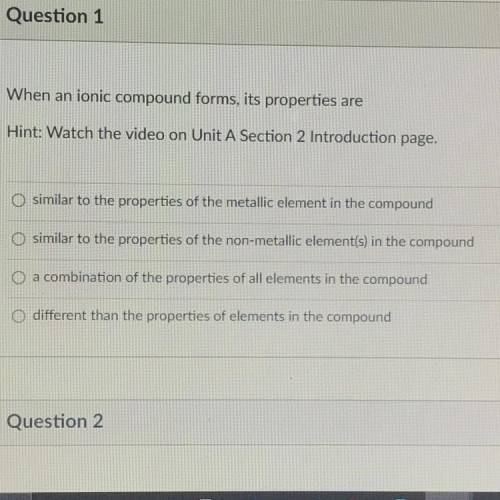
URGENT!! When an ionic compound forms, its properties are...
...

Chemistry, 01.06.2021 19:10 queenkalah101
URGENT!! When an ionic compound forms, its properties are...

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 21:00
In a buffer system of hf and its salt, naf, the f- neutralizes added base the hf is not necessary the hf neutralizes added base the hf neutralizes added acid the f- neutralizes added h2o
Answers: 3
You know the right answer?
Questions

English, 08.03.2021 20:40

Mathematics, 08.03.2021 20:40

Mathematics, 08.03.2021 20:40


Spanish, 08.03.2021 20:40

Mathematics, 08.03.2021 20:40

Mathematics, 08.03.2021 20:40

Physics, 08.03.2021 20:40


English, 08.03.2021 20:40

Mathematics, 08.03.2021 20:40


English, 08.03.2021 20:40

English, 08.03.2021 20:40



History, 08.03.2021 20:40



Mathematics, 08.03.2021 20:40



