Chemistry, 01.06.2021 16:00 dededese2403
112 g of aluminum carbide react with 174 g water to produce methane and aluminum hydroxide in the reaction shown below.
2 Al4C3(s) + 12 H2O(l) → 3 CH4(g) + 4 Al(OH)3(s)
If aluminum carbide is the limiting reactant, how many moles of the excess reactant are left over
a
37.3 mol
b
4.68 mol
c
7.33 mol
d
131.94 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

You know the right answer?
112 g of aluminum carbide react with 174 g water to produce methane and aluminum hydroxide in the re...
Questions

History, 15.11.2019 06:31

Mathematics, 15.11.2019 06:31

Chemistry, 15.11.2019 06:31



Mathematics, 15.11.2019 06:31

Computers and Technology, 15.11.2019 06:31



Mathematics, 15.11.2019 06:31



History, 15.11.2019 06:31

English, 15.11.2019 06:31

Mathematics, 15.11.2019 06:31


Mathematics, 15.11.2019 06:31

Mathematics, 15.11.2019 06:31


Physics, 15.11.2019 06:31

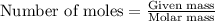 .....(1)
.....(1)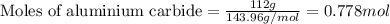
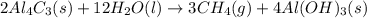
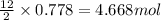 of water
of water