Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
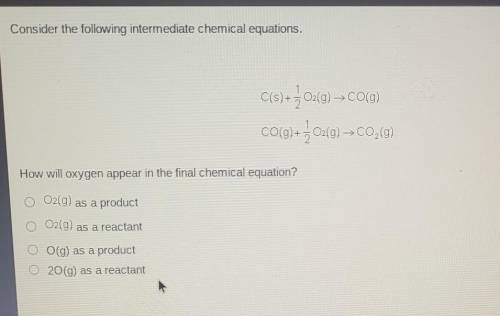
Consider the following intermediate chemical equations.
C(s) + + O2(g) → CO(g) CO(g) + } 02(g) C0,0...
Questions

Mathematics, 25.09.2019 05:30




Mathematics, 25.09.2019 05:30


Biology, 25.09.2019 05:30

Social Studies, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30

English, 25.09.2019 05:30





English, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30

Social Studies, 25.09.2019 05:30


Social Studies, 25.09.2019 05:30