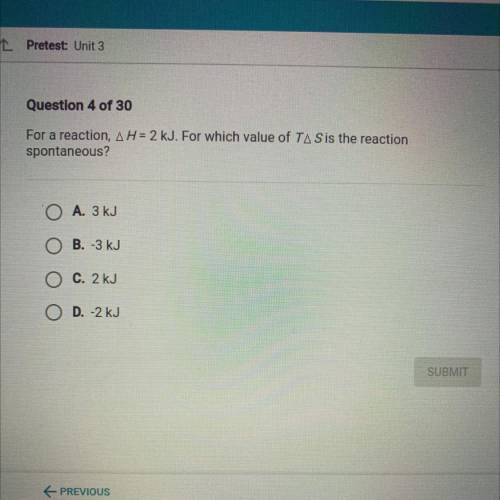
For a reaction, AH = 2 kJ. For which value of TA Sis the reaction
spontaneous?
...

Chemistry, 31.05.2021 22:00 moningersavannah
For a reaction, AH = 2 kJ. For which value of TA Sis the reaction
spontaneous?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
Questions

Mathematics, 12.10.2019 06:50

Mathematics, 12.10.2019 06:50



History, 12.10.2019 06:50

Arts, 12.10.2019 06:50

Mathematics, 12.10.2019 06:50

Social Studies, 12.10.2019 06:50

Mathematics, 12.10.2019 06:50



History, 12.10.2019 06:50

Social Studies, 12.10.2019 06:50


Mathematics, 12.10.2019 06:50


Mathematics, 12.10.2019 06:50


Mathematics, 12.10.2019 07:00

Physics, 12.10.2019 07:00



