

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Given the following reaction:
2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) ΔH = -927.54 kJ
...
...
Questions


Mathematics, 02.11.2021 22:40

Mathematics, 02.11.2021 22:40

Biology, 02.11.2021 22:40


Chemistry, 02.11.2021 22:40


Mathematics, 02.11.2021 22:40


Mathematics, 02.11.2021 22:40

Mathematics, 02.11.2021 22:40


Health, 02.11.2021 22:40






English, 02.11.2021 22:40

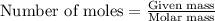 ......(1)
......(1)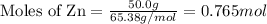
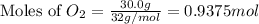

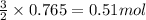 of oxygen gas
of oxygen gas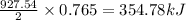 of energy
of energy

