
Chemistry, 29.05.2021 22:00 brianna8739
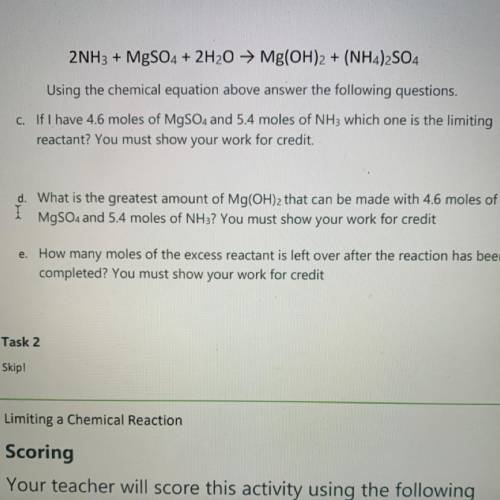
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following questions.
C. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of
MgSO4 and 5.4 moles of NH3? You must show your work for credit
e. How many moles of the excess reactant is left over after the reaction has been
completed? You must show your work for credit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3


Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following qu...
Questions

Social Studies, 26.10.2019 18:43


Mathematics, 26.10.2019 18:43



Biology, 26.10.2019 18:43

Computers and Technology, 26.10.2019 18:43



Mathematics, 26.10.2019 18:43


English, 26.10.2019 18:43


Mathematics, 26.10.2019 18:43

Mathematics, 26.10.2019 18:43

Mathematics, 26.10.2019 18:43


Biology, 26.10.2019 18:43


Social Studies, 26.10.2019 18:43



