Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2


You know the right answer?
A chemist mixes 50.0mL of a 1.0M NaOH solution with 50.0mL of a 1.0M Ba(OH)2 solution. Assuming the...
Questions



Spanish, 20.04.2021 08:10


Social Studies, 20.04.2021 08:10



Mathematics, 20.04.2021 08:10





Mathematics, 20.04.2021 08:10

Mathematics, 20.04.2021 08:10



Mathematics, 20.04.2021 08:10


Mathematics, 20.04.2021 08:10

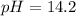
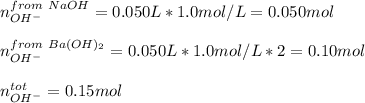
![[OH^-]=\frac{0.15mol}{0.100L}=1.5](/tpl/images/1354/5960/12bf6.png)