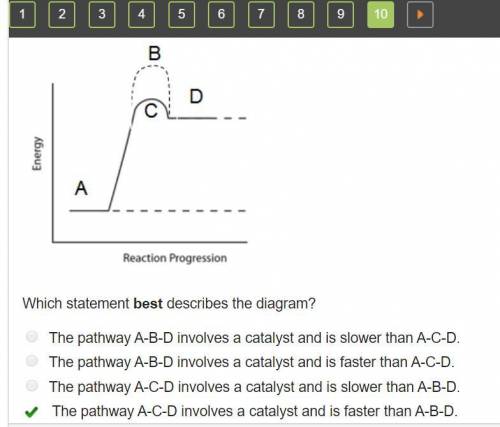
Consider the energy diagram below.
A graph of reaction progression on the horizontal axis versus energy on the vertical axis. A line starts flat low on the vertical axis, the flat area is labeled A. It rises sharply to peak, labeled C, then falls a short distance before levelling off, labeled D. A dotted line follows the initial line in all respects except that it peaks higher directly above C; that peak is labeled B.
Which statement best describes the diagram?
A. The pathway A-B-D involves a catalyst and is slower than A-C-D.
B. The pathway A-B-D involves a catalyst and is faster than A-C-D.
C. The pathway A-C-D involves a catalyst and is slower than A-B-D.
D. The pathway A-C-D involves a catalyst and is faster than A-B-D.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Consider the energy diagram below.
A graph of reaction progression on the horizontal axis versus en...
Questions

Mathematics, 22.07.2021 14:00

Social Studies, 22.07.2021 14:00



Mathematics, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00

Social Studies, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00


Mathematics, 22.07.2021 14:00


Mathematics, 22.07.2021 14:00

History, 22.07.2021 14:00

English, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00

Mathematics, 22.07.2021 14:00

Biology, 22.07.2021 14:00


Computers and Technology, 22.07.2021 14:00