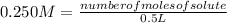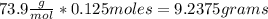Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

You know the right answer?
The molar mass of lithium carbonate is equal to 73.9 g/mol. Answer the following questions. 1) What...
Questions





Physics, 29.03.2021 17:40

Mathematics, 29.03.2021 17:40

Mathematics, 29.03.2021 17:40


Mathematics, 29.03.2021 17:40

Mathematics, 29.03.2021 17:40



Chemistry, 29.03.2021 17:40

Computers and Technology, 29.03.2021 17:40

Mathematics, 29.03.2021 17:40

Mathematics, 29.03.2021 17:40

Social Studies, 29.03.2021 17:40

English, 29.03.2021 17:40


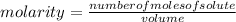
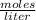 .
.